NADPH creation by the folate cycle
The folate cycle involves the conversion of dietary folate to different precursors involved in other metabolic pathways depending on cellular needs.
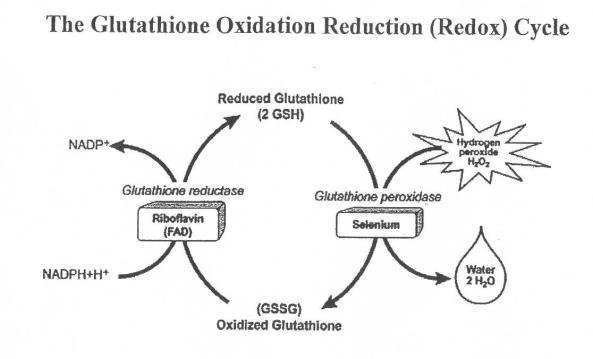
Folate is first converted to THF and then 5,10-MTHF where it enters a crossroads that is partially dictated by the NADP+:NADPH ratio. NADP+ works with the enzyme MTHFD to direct 5,10-MTHF toward the right in the diagram to help synthesize nucleotides for DNA. This is the pathway that generates NADPH and inhibition of the MTHFD enzyme increases the ratio of NADP+:NADPH as well as GSSG:GSH(2), shifting redox balance towards oxidation. As with MTHFR, there are mutations in the MTHFD gene that can negatively impact methylation, likely through changing cellular redox balance.
In the other direction, the enzyme MTHFR converts 5,10-MTHF to 5-MTHF which is used to help power the methylation cycle. In order for the pathway to operate in this direction, NADPH is necessary as MTHFR is an NADPH dependent enzyme. This is where it gets interesting. People with a mutation in the MTHFR gene have lowered activity of the MTHFR enzyme depending on whether they have one or two copies of one of the mutations. This causes the methylation cycle to function sub-optimally which causes a build up of homocysteine since they don't make sufficient levels of 5-MTHF to help convert homocysteine to methionine. These people experience reduced enzyme activity merely because they don't produce enough of the enzyme, but that's not the only way MTHFR activity can become reduced.
The activity of the MTHFR enzyme is also dictated by the availability of NADPH, so even people with no MTHFR mutation can experience poor methylation if the redox balance favors NADP+(Oxidation), especially if they have a mutation of the MTHFD gene. The problem with having reduced MTHFR activity either through a mutation or a less favorable redox balance is that homocysteine may increase free radical production directly(3), by reducing glutathione levels(4), or a combination of the two. It could also merely be an association where a high level of free radicals is indicative of a cellular environment that will produce more homocysteine, but recent evidence shows that high homocsyteine levels are at least partially causative in increased free radical production(5). This would promote a more oxidative environment and decrease methylation further. Maintaining a lower ratio of NADP+:NADPH via the pentose phosphate pathway should help promote a more reductive environment and increase methylation, but people with an MTHFR mutation still likely need to supplement with methylfolate to some extent.
In theory, people with an MTHFR or MTHFD mutation may be more prone to thiamine deficiency than people with the normal SNPs, or at the very least require more thiamine to maintain a more reductive cellular state. In the MTHFR mutation, if increasing levels of homocysteine change the redox balance to favor oxidative reactions, the pentose phosphate pathway will have to go in to overdrive in an attempt to restore a more reductive cellular environment. The same could be said with reduced activity of MTHFD since it is an NADPH generating pathway. Both would require more transketolase activity which will require more thiamine. Taken together, this could, in theory, mean people with an MTHFR or MTHFD mutation are more prone to adrenal fatigue, assuming the science linking thiamine status to adrenal function is correct.
Blood glucose, insulin resistance, and thiamine
As mentioned in my last blog, thiamine is used in more processes than the pentose phosphate pathway, specifically the pyruvate dehydrogenase and alpha-ketoglutarate dehydrogenase complexes in the mitochondria. If thiamine is tied up producing reducing equivalents in the pentose phosphate pathway in the cytosol, it may not make it to the mitochondria and could negatively impact the ability to generate energy from glucose there aerobically. Furthermore, since thiamine is transported in the blood and red blood cells utilize large amounts of thiamine in the pentose phosphate pathway because they are exposed to large numbers of free radicals, cells that utilize glucose may not get the thiamine they need to metabolize glucose for energy effectively when free radical levels are high, as in hyperglycemia. Decreased thiamine in cells that rely on oxidative glucose metabolism will decrease pyruvate dehydrogenase activity, increasing lactate production. This would increase blood lactate levels which is known to induce insulin resistance in skeletal muscle(6), forming a vicious cycle where free radical production induced by hyperglycemia increases insulin resistance and reinforces hyperglycemia, leading to more free radical production.This is not the only problem facing people with Type 2 diabetes. In an ironic twist of fate, Type 2 diabetics have altered thiamine metabolism. One study found marginally low plasma thiamine levels in people with Type 2 diabetes that translated in to reduced transketolase activity(7). Another study found plasma thiamine levels in people with Type 2 diabetes to be reduced by 75% compared to controls with a 16x increase in renal thiamine excretion(8). A yet to be published observational study found between 16%-29% of obese people seeking bariatric surgery to be deficient in thiamine(9) and a study in Australia found low plasma thiamine and folate in healthy blood donors(10), so this may not simply be an obesity/Type 2 diabetes problem but a problem of the modern diet. While the US healthcare system views thiamine deficiency as being relatively rare and only in alcoholics, it appears the data doesn't really support this position.
One of the more beneficial aspects of undertaking a Paleo diet is that it can have a profound effect on reversing Type 2 diabetes(11, 12). However, based on the available evidence, one may need to move from their old lifestyle to their new one with caution. If a person with Type 2 diabetes decides to move from the Standard American Diet to a Paleo diet and their inability to properly regulate blood glucose during their old diet put them at marginal or low thaimine status, cutting out grains and legumes, two significant sources of thiamine, may sink them deeper in to thiamine deficiency. Throw in a few days of glucose demanding Crossfit WODs per week and the stage for thiamine deficiency, and perhaps adrenal fatigue, is set. Supplementing with thiamine or making sure the diet provides adequate thiamine is the prudent course for those switching from a standard American diet to the Paleo diet.
Conclusion
At this point, I assume your head is spinning so let me break the last 2 blogs down for you. Cellular redox balance is important to cellular function as it helps dictate the direction of metabolic pathways. Two redox pairs exist in different ratios to allow anabolic and catabolic reactions to go on, sometimes at the same time, in a cell. The acronym ARCO can help you remember that anabolic reactions tend to favor reduction while catabolic reactions tend to favor oxidation. The low ratio of NADP+/NADPH favors reduction which makes it an anabolic coenzyme pair, but this balance can be thrown off if cellular free radicals rise and glutathione uses the reduction power of NADPH generated from the pentose phosphate pathway to maintain its ability to neutralize these free radicals. Low thiamine intake may also throw off cellular redox balance by reducing flux through the pentose phosphate pathway, reducing NADPH levels.Looking at the folate and methylation cycles, reduced activity of the MTHFR enzyme, which is dependent on NADPH, will increase free radicals through increased homocysteine production. Again, this would drive the redox balance towards oxidation via increased glutathione reduction. MTHFD, the enzyme responsible for directing the folate cycle in the opposite direction, is also of concern because it contributes significantly to the pool of NADPH in the cell. People with mutations in the genes that code for these enzymes likely have increased thiamine needs, particularly if their lifestyle leads to high levels of free radicals due to blood glucose fluctuations, high alcohol intake, or smoking.
Now, with the hard science over, in the next blog we can go over a more easy to understand mechanism by which thiamine deficiency can impact adrenal function: altered synthesis of the neurotransmitter acetylcholine.